Project Leaders:
Laura Santambrogio, MD, PhD / Dr Formenti , MD
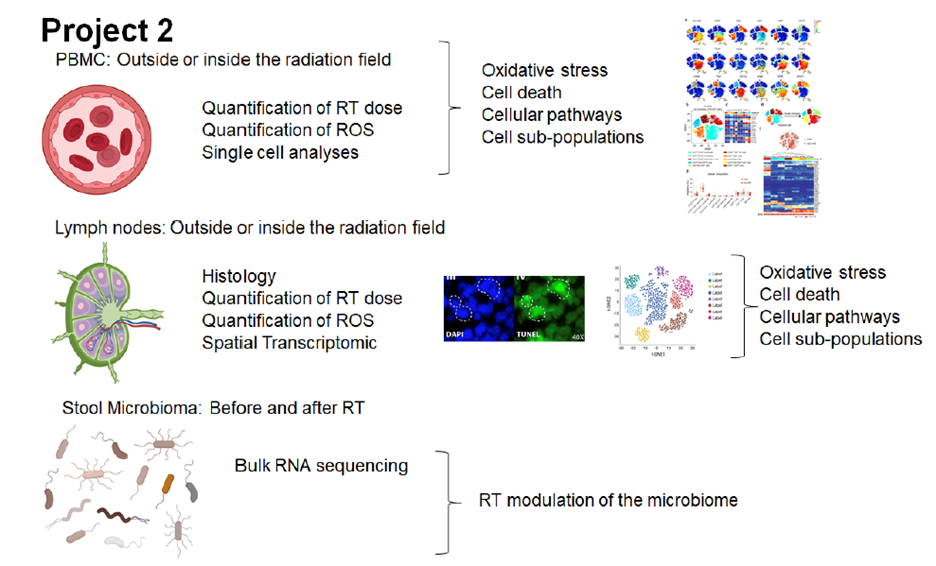
Over the last decades, we have enhanced our understanding of the molecular mechanisms that underlie the efficacy of RT and have also made progress to improve the efficiency of RT while reducing side effects associated with damage to nearby normal tissues. Nevertheless, a comprehensive understanding of how RT, in a given anatomical field, distinctively alters cancer cells, normal tissue, and infiltrating immune cells based on each cell’s “uniqueness" is still unresolved. Further, and importantly, how these combined RT-dependent effects on different cell types converge to influence therapeutic outcomes remains poorly understood. It is well recognized that immune fitness is important in controlling cancer growth and progression; as such, any therapy compromising immune cells, by default, can compromise the immune system’s ability to control malignant cells. Whereas Project 1 focuses on the tumor and tumor microenvironment response to RT in colorectal cancer, in this project (Project 2), we focus on the impact of RT on immune cells and the microbiome.
The overall hypothesis of Project 2 is that the immune system is an important component of the cancer response to RT and that innate and adaptive immune responses elicited by RT are pivotal to restrict cancer progression. However, these immunological benefits of RT are counterbalanced by deleterious effects of RT on immune cell fitness and survival with consequent leukopenia and immune dysfunction. As such, a quantitative understanding of the percentage and sub-populations of immune cells that are directly or indirectly (as bystanders) affected by RT-induced damage is fundamental to completely understand the role of RT in cancer treatment outcomes.
In Project 2, firstly, we will quantify the percentage of peripheral blood mononuclear cells (PBMC), as well as immune cells within lymph nodes located inside or outside the radiation field that are affected by RT. Secondly, we will quantify the RT-induced damage by calculating both the accumulated RT exposure at the single cell level and related cellular responses (i.e., apoptosis, ER mitochondrial stress, activation of inflammatory pathways, immunological fitness). Using single cell RNA-sequencing and spatial transcriptomics, we will generate a road map of the effects of RT on the different immune cell types (T and B cell subpopulations, dendritic cells, monocytes, macrophages, NK cells) in relation to RT dose exposure. Additionally, we will analyze qualitative and quantitative changes in the microbiome in samples collected before and after RT to infer how RT-mediated changes in the intestinal microbiome could affect immune responses. In the second part of Project 2, a machine learning approach will be utilized to integrate all of the acquired data to develop a more comprehensive view of how RT influences immune responses and treatment outcomes.



