Project Leaders:
Cristina Montagna, PhD / Lorenzo Galluzzi, PhD / Wen Shen, MD, MPH
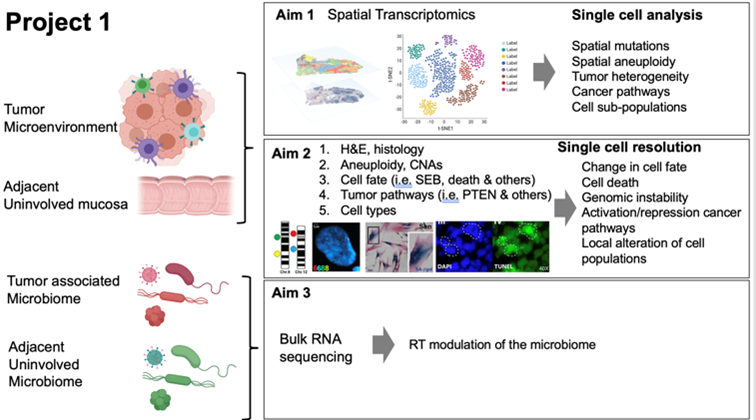
Summary:
Tumors develop in complex and dynamic microenvironments that influence disease progression and response to treatment, including radiation therapy (RT). RT results in compromised cellular fate as a consequence of unrepaired DNA lesions, macromolecular damage and failing adaptation to stress, culminating in cell death or permanent proliferative inactivation (cell senescence), both of which crosstalk with the immune system. Local differences in genomic instability, oxygenation and microbial components can also affect tumor responses to RT and immune activation. All these factors and their dynamic intertwining contribute to local control and patient survival. Despite the growing knowledge in radiation biology and the improved precision delivery of radiation to tumors, the molecular determinants of how RT impacts the tumor-immune interaction over time are still largely undefined. Moreover, how RT-induced stress response and adaptation in distinct cell components in the tumor microenvironment ultimately influence the clinical outcome remains elusive.
Likewise, how such therapeutic outcome can be further altered by distinct pre-existing signaling profiles related to genomic instability prior to RT, is not well characterized. Here, we test the novel hypothesis that pre-operative RT for resectable colorectal (CRC) cancer alters the dynamic interactions between malignant cells, normal cells, immune cells, and the tumor microbiome, to modulate cell fate decisions and anticancer immunity. Our team of basic scientists has joined forces with a group of immunologists, clinical oncologists, surgeons, pathologists, physicists, and bioinformaticians, to create a unique environment for testing this hypothesis in conjunction with Project 2. Specifically, we aim at (1) establishing the spatiotemporal landscape of the immune response and mutational and transcriptional changes within histological context induced by RT in CRC and the adjacent nonmalignant colon; (2) assessing the effect of RT on genome stability, cell fate, and modulation of cancer signaling pathways; and (3) investigating how RT modulates the tumor-associated microbiome. To these aims, we will harness a unique set of longitudinally collected CRC samples provided by the Molecular Characterization Trial (MCT) and state-of-the-art, innovative technologies including spatial transcriptomics (the technology of the year 2020), RNAscope and others, which will enable for an unprecedented (at near-to-single cell resolution) characterization of the dynamic interactions between malignant cells, normal cells, immune cells and the tumor microbiome in tumor samples, and how such interactions evolve prior to, during and after radiotherapy, with special emphasis on factors and processes that have previously been linked to radiosensitivity, including (but not limited to) genomic instability, hypoxia, DNA damage responses, cell death and cellular senescence.Tumors develop in complex and dynamic microenvironments that influence disease progression and response to treatment, including radiation therapy (RT). RT results in compromised cellular fate as a consequence of unrepaired DNA lesions, macromolecular damage and failing adaptation to stress, culminating in cell death or permanent proliferative inactivation (cell senescence), both of which crosstalk with the immune system. Local differences in genomic instability, oxygenation and microbial components can also affect tumor responses to RT and immune activation. All these factors and their dynamic intertwining contribute to local control and patient survival. Despite the growing knowledge in radiation biology and the improved precision delivery of radiation to tumors, the molecular determinants of how RT impacts the tumor-immune interaction over time are still largely undefined. Moreover, how RT-induced stress response and adaptation in distinct cell components in the tumor microenvironment ultimately influence the clinical outcome remains elusive.



